Weed management is essential during the establishment phase of orchards to improve the survival of newly transplanted nursery stock. In commercially bearing systems, weeds must be controlled in order to increase irrigation efficiency, provide equipment access, and ensure that fruits can be harvested effectively and economically. Furthermore, non-managed weeds may support populations of insect, vertebrate, and pathogenic pests that can significantly reduce tree health over time.
Weed control strategies may not always be 100% effective and escapes can occur for numerous reasons. Situations leading to herbicide failure include: improper herbicide selection or inappropriate timing of chemical applications, unfavorable weather conditions at the time of treatment, reduced herbicide activity due to poor water quality, and the development of herbicide resistance in the target weed population, among others. Plant size can also affect weed control; the efficacy of post-emergence herbicides is often diminished when products are applied to large/mature plants (Figure 1).
Figure 1. Ineffective management of large weeds in a plum orchard with a contact herbicide. Reduced control is attributed to poor herbicide coverage and subsequent plant regrowth.

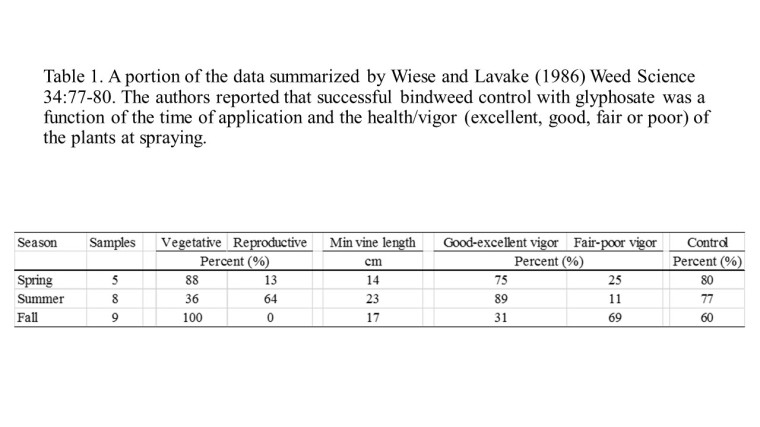
As an example: weed scientists at the University of California, Davis, recently undertook studies to describe how the control of hairy fleabane (Conyza bonariensis), a close relative of marestail/horseweed (Conyza canadensis), differed with respect to plant size at the time of herbicide application. Treatments included: glyphosate (Roundup WeatherMax at 3.5pt/A), glufosinate (Rely 280 at 3 pt/A), paraquat (Gramoxone Inteon at 3pt/A), and saflufenacil (Treevix at 1 oz/A) applied at one of three different growth stages: small rosette (4- to 5-leaf, < 1 inch height), large rosette (15- to 20-leaf, < 7 inches in height), and bolting (> 20 leaves, 7 inches in height).
Greater than 90% control of hairy fleabane was achieved when plants were treated with glyphosate, regardless of size at the time of application (Table 1). Glyphosate is a systemic herbicide that it is translocated within treated plants; it eventually accumulates at meristems and inhibits the production of aromatic amino acids (tryptophan, tyrosine, and phenylalanine) that are needed for protein synthesis. Conversely, the efficacy of glufosinate, paraquat, and saflufenacil decreased when hairy fleabane plants began to bolt (Table 1). Unlike glyphosate, these active ingredients exhibit no or limited mobility in treated plants; as a consequence, herbicide-induced injury is limited to the tissues that come into contact with the spray solution. In the case of larger plants, the outermost portions of the canopy can act as a shield for the innermost leaves, stems, and buds, which may continue growing. Additionally, contact herbicides will not impact underground tissues (roots, rhizomes, bulbs, etc.), which also support plant regrowth.
Table 1. Hairy fleabane control with glyphosate (Roundup WeatherMax at 3.5pt/A), glufosinate (Rely 280 at 3 pt/A), paraquat (Gramoxone Inteon at 3pt/A), and saflufenacil (Treevix at 1 oz/A) as affected by plant size at the time of application.

Still, it isn’t sufficient to focus on in-season weed control as the sole metric of efficacy. Plants that escape treatment may reach reproductive maturity and produce viable seeds. These seeds are the foundation for weed problems in future years. As a consequence, weed escapes necessitate that growers engage in additional management practices that may have been unplanned and that add to the cost of crop production. Increased seedbank/in-field weed densities could also facilitate the development of herbicide resistance. When weed infestations are heavy, the probability of selecting for resistance can be high, even if the mutation rate that leads to the development of resistance is low.
There are several steps that growers can take to maximize weed control with post-emergence herbicides, including timing applications to treat weeds while they are small and tender. Additional strategies include: selecting the appropriate herbicides to control target weed species and applying them at appropriate rates, minimizing off-target movement due to rain and wind, calibrating spray equipment, properly, and using adjuvants, effectively, to ensure coverage and penetration. To minimize the potential for herbicide resistance, growers should diversify their chemical, cultural, and physical weed control strategies as much as is environmentally and economically possible.
Any mention of herbicides in this blog does not constitute a professional recommendation by the author or her employers.