Field bindweed (Convolvulus arvensis L.), a deep-rooted and drought tolerant perennial, is a significant concern of the processing tomato industry in California. If allowed to compete with tomatoes during canopy establishment, field bindweed can significantly reduce both fruit number and quality. Furthermore, field bindweed vines can become physically entwined with tomato plants, which, in turn, can reduce harvest efficiency.
Although bindweed seedlings are relatively easy to manage using physical and chemical control strategies, established plants with extensive root systems are relatively tolerant to most management practices. For example, perennial bindweed control with tillage and cultivation is made more difficult by the weed’s significant below-ground nutrient reserves and regenerative capacity. Infrequent mechanical cultivation may also facilitate plant spread by dispersing root fragments as opposed to exhausting stored energy. Suppression of established bindweed using chemical tools may be equally challenging, especially in crops like processing tomato, where effective herbicide options are limited.
Post-emergence herbicides (applied as a pre-plant burn down, for post-harvest field cleanup, or used in-crop) can be important tools for managing field bindweed infestations. In 2013 and 2014, we conducted two trials at the University of California, Davis, research farm to evaluate the efficacy of glyphosate (as Roundup Powermax), rimsulfuron (as Matrix), and carfentrazone (as Shark) for the control of vigorously growing field bindweed vines. All herbicides were applied post-emergence (POST) using a CO2-pressurized backpack sprayer equipped with three 8002VS flat-fan nozzles (TeeJet Technologies, Wheaton, IL) spaced 16-20 in apart and calibrated to deliver 30 GPA. Adjuvants were used according to label recommendations. Control of field bindweed was rated for 3-5 weeks after application.
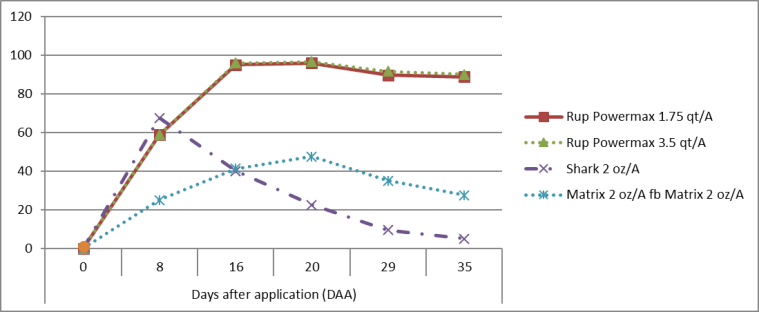
Figure 1. Bindweed control (%) in response to post-emergence applied herbicides (in field, 2013) for up to 35 days (or 5 weeks) after herbicide application (DAA). Herbicides: Roundup Powermax = glyphosate, Shark = carfentrazone, Matrix = rimsulfuron.

Figure 2. Bindweed control (%) in response to post-emergence applied herbicides (in field, 2014) at application and at 1, 2, and 3 weeks after treatment (WAT). Herbicides: Roundup Powermax = glyphosate, Shark = carfentrazone, Matrix = rimsulfuron.
Results presented in Figures 1 and 2 show that POST herbicide applications of rimsulfuron and carfentrazone were largely ineffective at controlling field bindweed. In the 2013 trial, Field bindweed control with rimsulfuron did not exceed 50%; in 2014, rimsulfuron was unable to provide more than 20% control at any observation date. Carfentrazone will burn down aboveground bindweed vines, giving the appearance of effective management, although regrowth can rapidly occur. In our research trials, carfentrazone controlled field bindweed 60 and 95% at 1 WAT; however, within 3-5 WAT, control fell to 5 and 40%. Glyphosate is slower to demonstrate activity, although its suppressive ability may persist for a longer period of time.
A similar study was conducted in 2016 (Table 1), except that estimates of bindweed cover, plant vigor (on a scale of 1-5, where 1 = poor and 5 = excellent) and the percentage (%) of vines that were producing flowers were determined instead of control. The untreated check plots produced more cover (40-73%) at 1, 3, and 5 WAT as compared to the glyphosate (23-50%) and rimsulfuron (32-43%) treated plots; the higher rate of glyphosate was more effective at suppressing bindweed (25-25% cover) than the lower rate (23-50%). Bindweed vigor in the untreated plots ranged between 3 and 3.5 at every observation date. The vigor ratings for the glyphosate and rimsulfuron treatments ranged from 3-4 at 1 WAT to 1-2.2 at 5 WAT. Reductions in vigor were associated with chlorosis and necrosis of leaf and stem tissue. Field bindweed flowering was also affected by POST herbicide treatments. Few vines exhibited any flowers, regardless of treatment on 18 May. On 1 June and 14 June, 42 and 50% of the vines untreated check plots were flowering; conversely, less than 7% of the vines in the glyphosate or rimsulfuron treatments were flowering at the same observation periods.
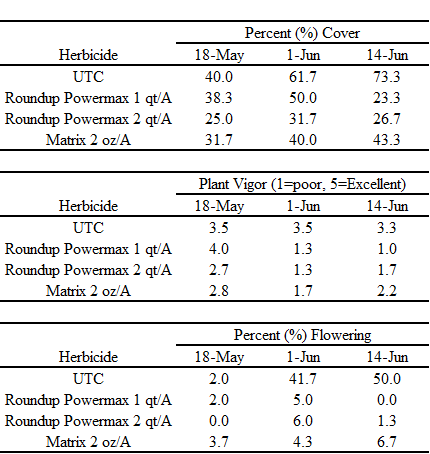
Table 1. Field bindweed cover (percent (%) of plot area covered with vines), plant vigor, and percent (%) of vines with flowers in response to post-emergence (POST) herbicide applications at approximately 1 (18 May, 3 (1 June), and 5 (14 June) weeks after treatment (WAT). Roundup Powermax = glyphosate, Matrix = rimsulfuron, UTC = untreated.
A comparable trial was also conducted in the greenhouse (Tables 2, 3, 4). Results show that bindweed injury, growth, and biomass accumulation were more affected by glyphosate than by rimsulfuron and carfentrazone. The injury observed in the rimsulfuron and carfentrazone treatments was more severe than what had been witnessed, previously in field trials. This is likely due to the fact that the field bindweed plants used in the greenhouse had been grown from exhumed rhizomes and did not possess the ample storage reserves that large, field-grown patches are expected to have. As has been described, previously, the highest rate of glyphosate was the most effective treatment for injuring field bindweed and suppressing plant growth.

Table 2. Number of field bindweed vines greater than 4 inches in length and length in cm of the longest vines at the time of application and at 28 days after treatment (DAT) in response to post-emergence (POST) herbicide applications. Roundup Powermax = glyphosate, Shark = carfentrazone, Matrix = rimsulfuron, UTC = untreated.
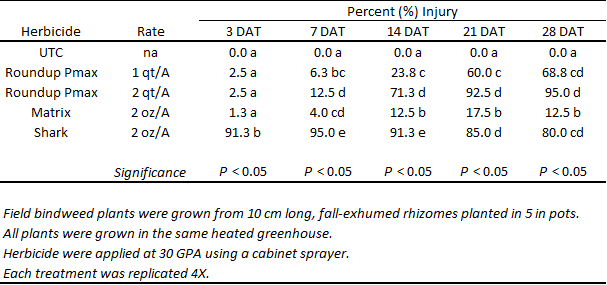
Table 3. Field bindweed injury at 3, 7, 14, 21 and 28 days after treatment (DAT) in response to post-emergence (POST) herbicide applications. Roundup Powermax = glyphosate, Shark = carfentrazone, Matrix = rimsulfuron, UTC = untreated.
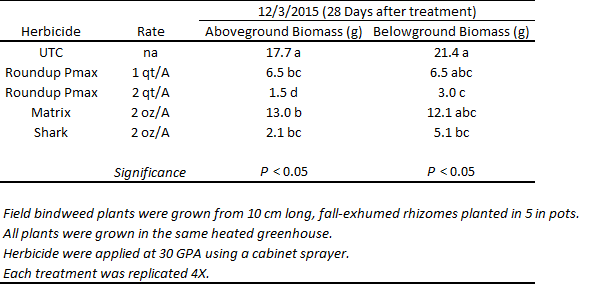
Table 4. Above and below ground field bindweed biomass at 28 days after treatment (DAT) in response to post-emergence (POST) herbicide applications. Roundup Powermax = glyphosate, Shark = carfentrazone, Matrix = rimsulfuron, UTC = untreated.
It is important to recognize that the timing of herbicide applications can also significantly affect weed control performance. For example, numerous growers, commercial applicators, and university personnel have reported that the performance of several herbicides (i.e. glyphosate, glufosinate, paraquat) may fluctuate with respect to application time of day (diurnally). Possible factors influencing herbicide performance include: circadian changes in leaf angle that affect herbicide interception; differences in humidity and temperature that may affect herbicide absorption and translocation; the presence of dew, which can reduce herbicide retention; and physiological processes that may be affected by lack of sunlight.
In 2016, we conducted trial to evaluate if the herbicidal efficacy glyphosate (as Roundup Powermax at 1 and 2 qt/A), rimsulfuron (as Matrix at 2 oz/A), carfentrazone (as Shark at 2 oz/A), and paraquat (as Gramoxone Inteon at 3 pt/A) varied with the time of day the herbicides were applied. Herbicides were applied to vigorously growing bindweed vines on 29 June, 2016, at five different times during the day: sunrise, 2 hours after sunrise, mid-day, 2 hours before sunset, and at sunset. All herbicides were applied using a CO2-pressurized backpack sprayer equipped with three 8002VS flat-fan nozzles (TeeJet Technologies, Wheaton, IL) spaced 16-20 in apart and calibrated to deliver 30 GPA. Field bindweed cover (percent of the plot area covered with field bindweed vines) was rated for 5 weeks after the treatments were applied (WAT).

Table 5. Field bindweed cover for up to 5 weeks after treatment (WAT), which occurred on 29 June, 2016, in response to post-emergence (POST) herbicide applications made at sunrise, 2 hours after sunrise, midday, 2 hours before sunset, and sunset. Herbicides: Roundup Powermax = glyphosate, Shark = carfentrazone, Matrix = rimsulfuron, Gramoxone Inteon = paraquat, UTC = untreated.
Results from the diurnal timing trial (Table 5) indicated that herbicide, alone, had a significant effect on bindweed cover; the timing of herbicide applications and the interaction between herbicide and the time of day when the herbicides were applied were not significant. Although the carfentrazone and the paraquat treatments worked rapidly (field bindweed cover was reduced by more than 90% at 1 WAT), the vines regrew vigorously; cover at 5 WAT was 45 and 42%, which was similar to what was observed for the untreated check (37%). Glyphosate, while slower acting due to its systemic nature, was significantly better at reducing field bindweed cover at 5 WAT (2 and 5%) relative to the untreated check. In this trial, rimsulfuron did not reduce field bindweed cover, relative to the control, at any point in time. Bindweed cover in the untreated check and the rimsulfuron treatment decreased over time due to the development of powdery mildew, which resulted in leaf loss. Interestingly, the bindweed regrowth in the carfentrazone and paraquat treatments was robust and did not show signs of infection during the course of this study.
Rimsulfuron is labeled for POST use in California processing tomatoes (http://ipm.ucanr.edu/PMG/r783700311.html) although its efficacy as a POST herbicide, with respect to field bindweed control, is poor. Paraquat and carfentrazone are both labeled for the control of emerged weeds prior to transplanting, although perennial weed control will not be long lasting as both products are contact herbicides and will do little more than burn off any above ground foliage. Glyphosate, was the most effective POST product for suppressing field bindweed growth across all trials; it is labeled for use as a pre-plant burn down. One of the tenets of integrated pest management is to start off clean and remain clean to prevent weed interference in the current and future crops. Growers with significant bindweed problems should strive to ensure effective burn down of existing vines prior to crop planting and following harvest; the management of bindweed in rotation crops and following rotation crop harvest is also encouraged.
The mention of herbicides in this post does not constitute a professional recommendation by the author or her employers.




